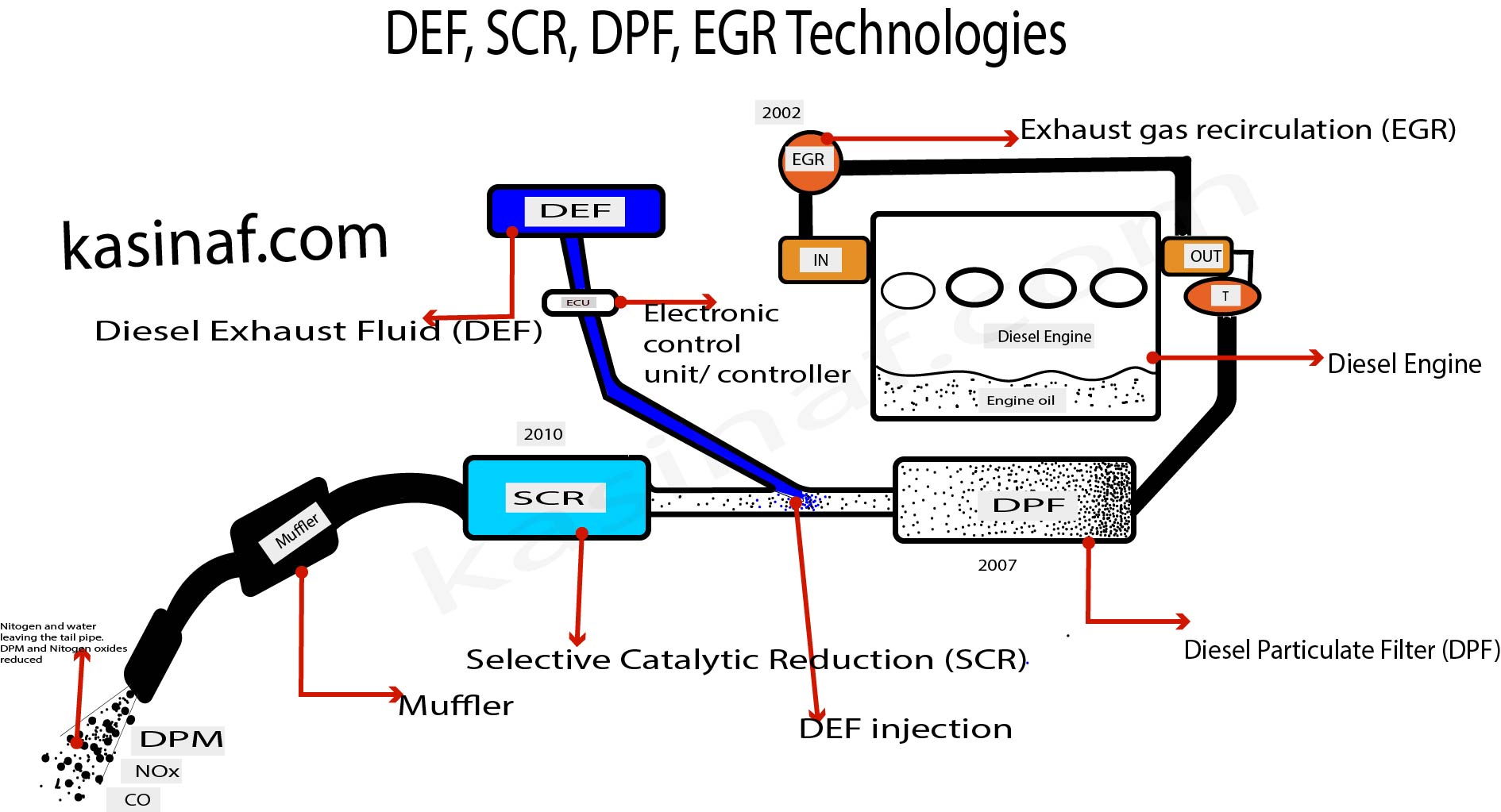
Diesel Exhaust Fluid (DEF) is a precisely blended mixture of two main components: synthetic urea and de-ionized water. The most common formulation of DEF consists of approximately 32.5% high-purity urea and 67.5% de-ionized water. The urea used in DEF is typically of automotive or vehicle-grade quality to ensure its effectiveness and compatibility with the Selective Catalytic Reduction (SCR) systems in diesel engines.
The DEF solution is clear, colorless, and non-toxic. It has a slight odor similar to ammonia due to the ammonia content that is formed during the chemical reactions within the SCR system.
DEF is a regulated fluid, and its composition must meet the quality standards defined by the American Petroleum Institute (API) under the DEF standard ISO 22241. It is important to use high-quality DEF to ensure proper functioning of the SCR system and to meet emissions regulations.
DEF is stored in a separate tank on diesel vehicles, and it is injected into the exhaust stream to help reduce harmful nitrogen oxide (NOx) emissions from diesel engines, as discussed earlier.
Effects of nitrogen oxide emissions
DEF is used in diesel engines to reduce harmful nitrogen oxides (NOx) emissions. NOx is responsible for various issues, including health problems when inhaled, the formation of nitric acid, and the creation and destruction of ozone. By using DEF in diesel engines, the formation of NOx is significantly reduced, leading to cleaner air and improved environmental conditions in areas where people live and work.
Chemical reactions of DEF
Let’s break down each of the equations and understand the chemical reactions involved in the process of Diesel Exhaust Fluid (DEF) decomposition and its role in reducing harmful emissions.
- DEF Becomes Ammonia and Isocyanic Acid: (NH2)2CO → NH3 + HNCO
In this first step, DEF, which is represented by the chemical formula (NH2)2CO, undergoes decomposition when heated. It breaks down into two substances: ammonia (NH3) and isocyanic acid (HNCO).
- Isocyanic Acid Reacts with Water to Produce Carbon Dioxide and Ammonia: HNCO + H2O → CO2 + NH3
The isocyanic acid (HNCO) produced in the previous step reacts with water (H2O). This reaction results in the formation of carbon dioxide (CO2) and ammonia (NH3).
Overall:
(NH2)2CO + H2O → 2NH3 + CO2
The overall reaction for the combination of Step 1 and Step 2 shows that DEF, when exposed to water, breaks down into two molecules of ammonia and one molecule of carbon dioxide.
- Ammonia Reduces Nitrogen Oxides (NOx) in the Presence of Oxygen and a Catalyst:
2NO + 2NH3 + ½O2 → 2N2 + 3H2O 3NO2 + 4NH3 → 7/2N2 + 6H2O
In these reactions, the ammonia (NH3) produced in the earlier steps acts as a reducing agent. It reacts with nitrogen oxides (NOx), represented by NO and NO2, in the presence of oxygen (O2) and a catalyst. As a result, the nitrogen oxides are converted into harmless nitrogen gas (N2) and water vapor (H2O).
Overall:
2(NH2)2CO + 4NO + O2 → 4N2 + 4H2O + 2CO2 2(NH2)2CO + 3NO2 → 7/2N2 + 4H2O + 2CO2
These overall reactions show the reduction of nitrogen oxides (NOx) when DEF reacts with them. The combination of DEF, nitrogen oxides, oxygen, and a catalyst results in the formation of nitrogen gas, water vapor, and carbon dioxide.
In summary, Diesel Exhaust Fluid (DEF) plays a crucial role in reducing harmful emissions, particularly nitrogen oxides (NOx), in the exhaust gas of diesel engines. When DEF is injected into the hot exhaust gas, it breaks down into ammonia and isocyanic acid. The ammonia then reacts with nitrogen oxides, effectively reducing them into non-hazardous nitrogen and water vapor, thereby helping diesel engines meet emissions regulations and contribute to cleaner air.